◆ 会议时间:2024年3月3-7日
◆ 会议地点:新加坡(Singapore)
◆ 会议简介:
2024年第18届世界麻醉医师大会(WCA 2024)将于2024年3月3-7日在新加坡举行,会议由世界麻醉师学会联盟(WFSA)主办。
世界麻醉医师学会联盟(WFSA)成立于1955年,现拥有来自150多个国家的134多个麻醉师协会成员。WFSA致力于联合全世界的麻醉医师,提高患者的护理水平,让每个患者都能获得安全的麻醉治疗。世界麻醉师协会联盟(WFSA)每四年会和一个国家协会成员联合举办一次世界麻醉师大会(WCA),世界麻醉师大会(WCA)为促进科学、教育、培训以及麻醉学各个领域的经验交流提供了一个难得的机会。——未经许可,禁止复制摘录转载本站任何内容-领域国际医学会议网。
WCA 2024 - 18th World Congress of Anaesthesiologists
Date: 3-7 March 2024
Venue: Singapore
Organized by: World Federation of Societies of Anesthesiologists
The World Congress of Anaesthesiologists (WCA) is the pre-eminent international congress for anaesthesiologists.
Bringing together anaesthesia and perioperative care providers from around the world, WCA provides an extraordinary opportunity for the promotion of science, education, training, networking and the exchange of experience across the spectrum of anaesthesiology.
With delegates from 130+ countries the WCA is unique in terms of global reach and diversity and offers an outstanding scientific and social experience for all who attend.
摘要征文投稿:
Important Deadlines and More Information
点此提交摘要>>>Submit Abstract>>>
点此查看更多摘要提交相关信息>>>
Abstract Submission Guidelines
Abstracts can only be submitted online via the online abstract submission system. Abstracts sent by post or email will not be accepted. No exceptions will be made.
Abstracts can be submitted if the following applies:
- Abstracts having been submitted for publication or to a scientific meeting, but are pending acceptance, can be submitted without restrictions. However, once the abstract has been accepted by WCA2024 it is automatically subject to the WCA2024’s terms and conditions.
Research abstracts should be structured into the following sections:
- A title which clearly indicates the nature of the investigation. If the research involves animals, state the animal species in the title of the abstract.
- Background & Objectives
- Methods
- Results
- Discussion and Conclusion
- References
For submission of Research abstracts, you are required to submit the Institutional Review Board’s approval/waiver letter and the trial registration number.
Case Studies should be structured into the following sections:
- A title which clearly indicates the nature of the investigation.
- Background (What is the unique or significant problem highlighted by this case?)
- Case Description
- Discussion
- Learning Point (What is the take home message from this report?)
- References
For submission of Case Studies, you are required to declare whether you have obtained the patient’s consent for submitting their case study as an abstract.
Word Limit:
The acceptable length of the abstract is not more than 500 words. This does not include references.
References can be up to 100 words.
Graph/Image:
One graph/image can be included.
Table:
One table can be included.
Language:
Only English is accepted. Please ensure that your abstract does not contain spelling, grammatical or scientific errors, as it will be reproduced exactly as submitted. No proofreading will be done.
注册费:
In-Person
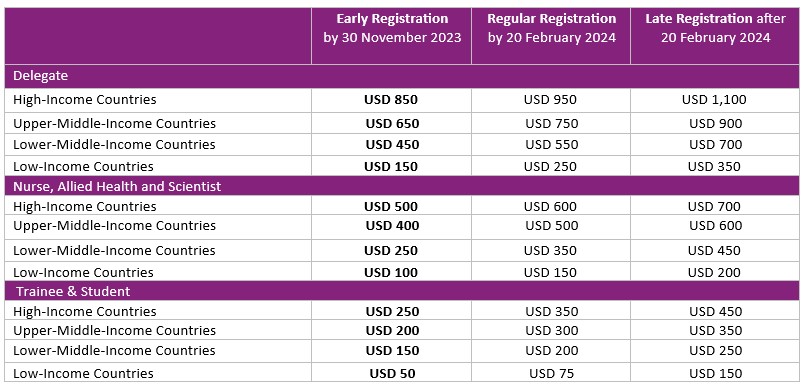
In-person registration fee includes:
- Access to all Scientific Sessions (except ticketed sessions and workshops)
- Access to Industry Symposia
- Access to Exhibition Hall
- One ticket to the Welcome Reception on Monday 4 March 2024
- Access to Networking Breaks
- Access to on-demand session recordings held in the Plenary Room and to session recordings (audio and slides) from other session rooms within 24 hours of live broadcast and up to 3 months following the broadcast
- Certificate of Attendance
- CME Certificate (if required)
Please note lunches will be available for purchase at the Congress venue.
Virtual
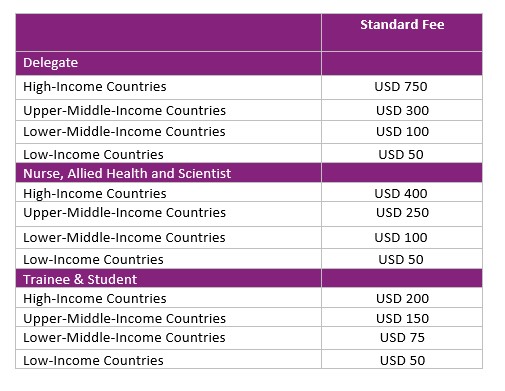
Virtual registration fee includes:
- Live stream (video and audio) of scientific sessions held in the Plenary Room and access to on-demand recordings
- Access to on-demand session recordings (audio and slides) from other session rooms within 24 hours of live broadcast and up to 3 months following the broadcast
- Certificate of Attendance
- CME Certificate (if required)
◆ 参会对象:医生、医院科室主任/副主任、住院医师、医院管理者、医护人员以及从事该领域研究的科学家、研究人员、医药企业代表等等。
|

