◆ 会议时间:2025年9月15-19日
◆ 会议地点:奥地利 维也纳
◆ 会议简介:
2025年第61届欧洲糖尿病研究协会(EASD)年会将2025年9月15-19日在奥地利维也纳举行。EASD年会是欧洲规模最大、最负盛名的糖尿病会议,每年在欧洲不同的城市举办。EASD年会科学计划为研究人员和医疗保健专业人士提供了一个分享想法和了解前沿的研究和技术、突破性研究成果以及糖尿病研究、治疗和护理方面重大进展的机会。EASD2025预计将吸引来自全球130多个国家的15000余名代表参会。
欧洲糖尿病研究协会(EASD)成立于1965年,是一个非营利性医疗科学协会,总部位于德国杜塞尔多夫。EASD致力于促进和支持糖尿病领域的研究,快速普及已获取的知识并促进其应用。目前EASD在全球100多个国家拥有超过5000名活跃会员,是世界最大的糖尿病专家网络之一。未经许可禁止复制摘录转载本站任何内容-国际医学会议网(lingyuint.com)。
EASD 2025 - 61st Annual Meeting of the European Association for the Study of Diabetes
Scientific Programme
Tuesday, 16 September - Friday, 19 September 2025
Venue:
Reed Messe Vienna
Messeplatz 1, 1021 Vienna, Austria
摘要征文投稿:
The EASD Session Proposal Submission System is now available via MyEASD点此提交 and will close on the deadline 28 April 2025 at 18:00hrs CEST.
点此登录提交
It is not permitted to submit work that has been published and/or is likely to be published before the EASD Abstract submission deadline (1 April 2025, 18:00hrs CEST). The Programme Committee has the right to remove an abstract if this contains data that has already been published. However, EASD accepts the submission of abstracts that have been presented in recent months at local or national diabetes meetings (e.g. ADA, DDG). An author submitting or presenting work that has been published in a journal before the EASD Abstract submission deadline (1 April 2025, 18:00hrs CEST) will be banned from presenting data at the EASD Meetings for three years.
Selection of abstracts for the Scientific Programme is made anonymously.
Your abstract submission must adhere the following structure:
- The maximum number of characters is 3,200 (excl. spaces) including the title and the author block. The title should be short (maximum 170 characters).
- Please enter your name and the names of co-authors as given in the example below. Start each name with a capital letter and continue in lower case, do not enter names/affiliations all in capital letters.
First Name: John
2nd Initial: J
3rd Initial: R
Last Name: Macleod
- The abstract must be structured. Enter each section into the corresponding text field with the provided sub-headings in bold (Background and aims, Materials and methods, Results and Conclusion). The abstract structuring words are fixed for each abstract text box. One or two sentences should describe the methods, and any aspects of methodology (e.g. use of control groups, randomisation, patient selection, assay variation). The sentences stating the results must include hard data, including statistical analysis. The abstract requires solid data, i.e. n, p-values and confidence intervals if appropriate.
- It is allowed to include only one table OR one graph to your abstract, but 400 characters will be deducted from the maximum number of characters allowed. Tables must be uploaded as an image; so you must create your table and convert it into an image in order to include it in your abstract. You can edit your table or graph and then replace it, or delete it until the submission deadline of 1 April 2025, 18:00hrs CEST. Do not enter both (a table and a graph) in the same image file; also, do not create and upload multi panel images (a maximum of two panels is allowed). When creating a table, do not exceed a number of 10 columns and 12 rows with a maximum of 1 line per row!
- References are not allowed.
- For drugs exclusively generic names should be used (no trademarks).
- A list of approved abbreviations and units of expression for use without definition appears in Diabetologia.
- Grant/Support information must be entered in its designated field, not in the abstract body.
- Where applicable, the Clinical Trial Registration Number must be entered into its designated field, not in the abstract body.
- Only one keyword can be selected.
- Abstracts CANNOT be submitted as an e-mail attachment.
Once all submission steps have been successfully completed and the abstract deadline has passed, a notification confirming the final version of the abstract submission will be posted on the presenting author’s Scientific Submission portal (accessible by loging into MyEASD). All notifications will be posted ONLY on the presenting = first named author’s Scientific Submission portal, the name, middle name, last name and e-mail contact used during the submission process must match any existing EASD membership and/or MyEASD account (only one e-mail address per presenting = first named author).
In case of questions, or if no notification confirming the final version of the abstract submission has been received by 14 April 2025, please contact the EASD office: abstracts@easd.org
Keywords
One keyword from the list available here must be selected for the submission:
Scientific Programme Committee
Preference for oral presentation or short oral discussion is not possible, they rank equally. The Scientific Programme Committee allocates the accepted abstracts to either an oral presentation or a short oral discussion session. The Scientific Programme Committee Members will meet in May 2025 and have the absolute right to accept or reject abstracts. Their decision is final. Only accepted abstracts will be published in the Abstract Volume.
The Scientific Programme Committee’s decision will be posted ONLY on the presenting = first named author’s Scientific Submission portal, the name, middle name, last name and e-mail contact used during the submission process must match any existing EASD membership and/or MyEASD account (only one e-mail address per presenting = first named author).
Oral and Short Oral Presentations during the EASD Annual Meeting will be delivered live; will also be made available on the EASD Media Centre.
EASD late-breaking abstract submission 2025
The EASD late-breaking Abstract Submission System will be available via MyEASD from 3 June and will close on the deadline 17 June at 18:00hrs CEST.
The late-breaking abstract deadline is not an extension of the abstract submission deadline.
EASD2025 注册费
Registration fees
EASD is a non-profit organisation and as such not subject to VAT which also applies to the registration fees.
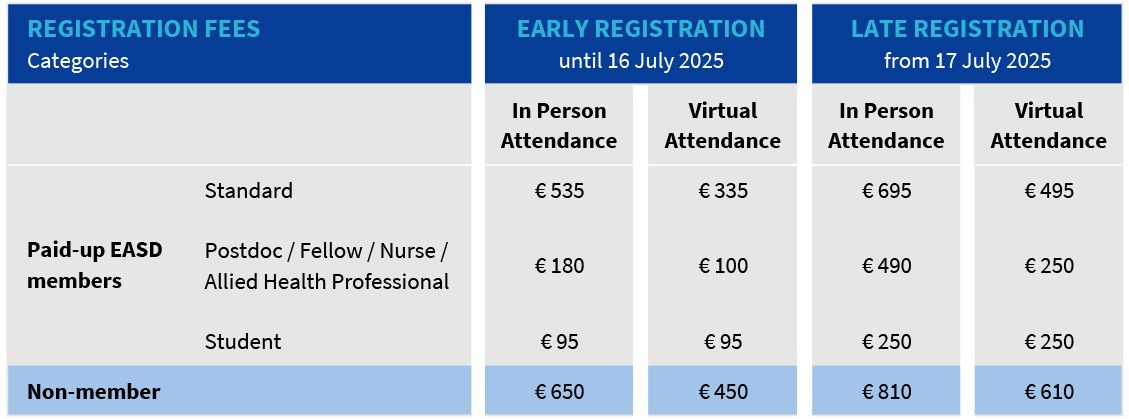
In Person attendees receive:
- Name Badge / Congress Material
- Access to the Scientific Programme
- Access to the Exhibition
- Access to the Virtual Meeting Platform for the duration of the meeting as well as the following 4 weeks after the meeting (incl. live and on demand sessions, Industry content)
- Certification based on the attendee`s in person and / or virtual attendance
- Public Transportation Ticket
Virtual attendees receive:
- Access to the Virtual Meeting Platform for the duration of the meeting as well as the following 4 weeks after the meeting (incl. live and on demand sessions, Industry content)
- Certification based on the attendee`s individual watch time
EASD2025 - 61st EASD Annual Meeting
Date: Tuesday, 16 September - Friday, 19 September 2025
Venue: Reed Messe Vienna Messeplatz 1, 1021 (Vienna, Austria)
◆ 参会对象:医生、医院科室主任/副主任、住院医师、医院管理者、医护人员以及从事该领域研究的科学家、研究人员、医药企业代表等等。
|

